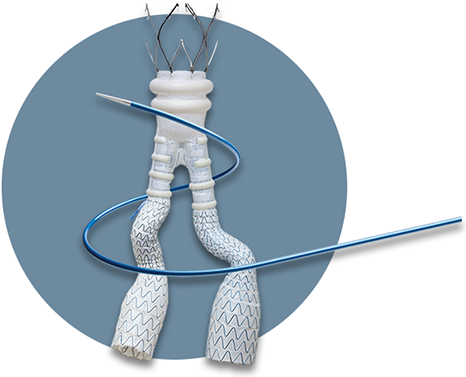
Endologix, based in Irvine, California, won clearance in the European Union for its ALTO Abdominal Stent Graft System. The implant is intended to open up endovascular aortic repair to a wider range of abdominal aortic aneurysm (AAA) patients, particularly those with short and challenging aortic necks.
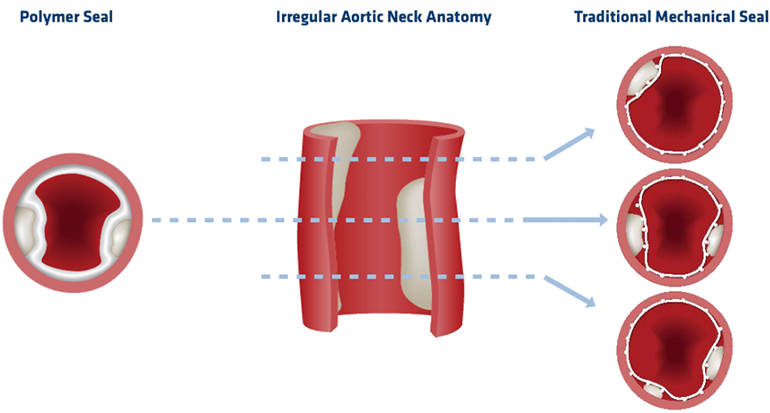
Ideally, the aortic neck where the stent graft creates a seal is regular and smooth. In many cases, this is simply not so and a conventional mechanical seal doesn’t work sufficiently. The ALTO features a novel polymer seal that seals tightly even irregularly shaped lumens, and which has been shown to keep a stable neck diameter five years post implantation, according to the company.
“We are very excited to receive a CE Mark for the ALTO system, that has been achieved through a strong partnership and collaboration with our European notified body, NSAI ” said Matt Thompson, CMO of Endologix, in the announcement. “ALTO will provide our physician partners and patients in the EU with a differentiated low profile endovascular treatment option designed to improve durability over traditional EVAR. In addition, with ALTO, we anticipate observing improved short-term outcomes relative to the Ovation iX Abdominal Stent Graft System (Ovation iX) as a result of the design and manufacturing changes incorporated into ALTO.”
“ALTO offers a highly differentiated endovascular treatment option to AAA patients and includes design features that we believe will enhance ease-of-use, improve acute outcomes and preserve the long-term durability associated with patient-specific anatomically adaptive sealing,” added John Onopchenko, CEO of Endologix. “We believe ALTO, with its ultra-low profile and 7mm aortic neck length indication, will provide patients and physicians with an endograft capable of treating the highest proportion of patients within the indications for use of the device. As we continue with the global roll-out of the ALTO system, which has commenced in the U.S., Endologix is committed to investing in the highest levels of clinical evidence by initiating a head-to-head randomized controlled trial versus traditional undifferentiated EVAR grafts, with the intent of proving the superiority of ALTO.”

Some of the system features, according to Endologix:
- Seal higher at 7 mm for challenging necks
- Improve sealing ring apposition and simplify procedure with integrated balloon
- Help reduce late failures with durable, suture-free construction
- Enhance stability with webbing at bifurcation
- Improve visibility with offset leg lengths
- Ultra low-profile 15F OD delivery catheter facilitates deliverability through narrow, diseased vessels
- Low-profile design intended to reduce vessel trauma
- Flexible delivery catheter resists kinking to ease maneuverability
- Enhance flexibility with helical nitinol stent iliac limbs
- Help reduce risk for occlusion with smooth, fully laminated PTFE fabric
- Flared limbs up to 28 mm diameter
- Limb lengths up to 160 mm
Flashbacks: Endologix Ovation Alto Abdominal Stent Graft Implanted In First Patients; Endologix AFX Endovascular AAA System FDA Approved; Endologix Device for Percutaneous Endovascular Treatment of AAA Begins Trial; Endologix Nellix Endovascular Graft for Abdominal Aortic Aneurysms; Endologix Gets Green Light to Market More Powerlink AAA Stent Graft Sizes
Via: Endologix
